

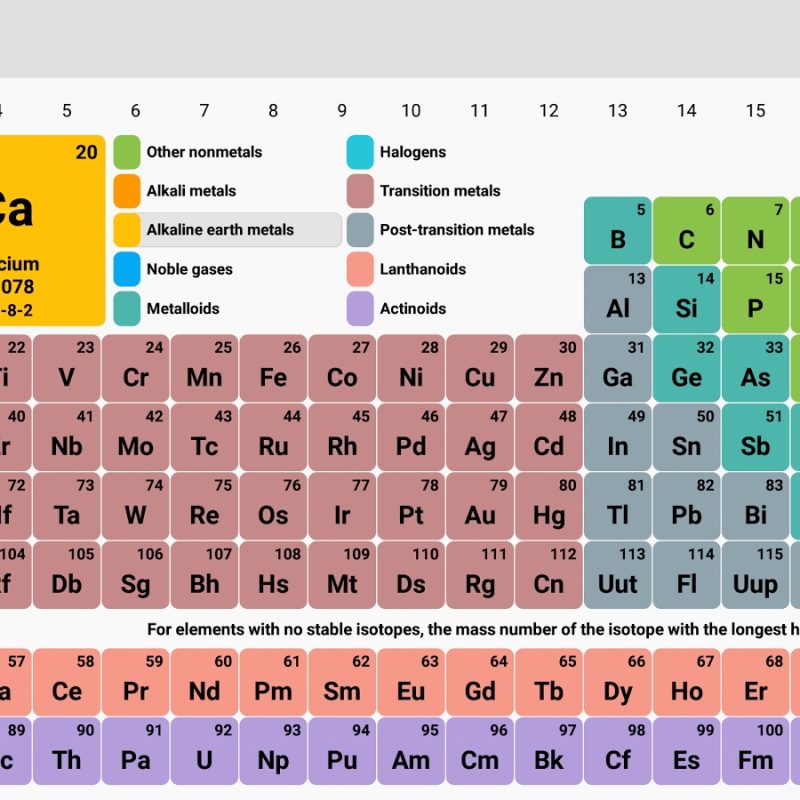
The elements that have identical chemical characteristics are assigned a group name and colored the same color. Rows and columns are called periods and groups. Periodic table consists of cells and each cell represents an element.
#Periodic table pdf how to
Only few people know about these elements because they are unstable and decay rapidly.ĭownload Template (196 KB) How to use a printable periodic table? They were placed at the seventh row off the table.
/PeriodicTable-White-58b5d8c15f9b586046df020c.png)
Some of them were officially recognized in 2015. Since the 1940s, scientists discovered he elements that are numbered 104- 118. However, at that time scientist rejected his suggestion but later research proved he was right.
#Periodic table pdf series
In 1945, he published a paper in which he said that actinide series of elements should be placed below the lanthanide series. Seaborg was working on the Manhattan project he hypothesized that additions needed to be made to the periodic table. During the middle of 20th century, when scientists discovered protons then an element assigned atomic number on the basis of number of protons it contains. He published the 18 column periodic table in 1923. You may also like course syllabus template. And, second it allowed the future scientists that without damaging the integrity of table, they can adjust the position of elements. The first one is that he allowed the placing of new elements and groups of elements he didn’t predicted.
This definition was used for 3 years until subatomic particles were discovered.
He states that it can be something that cannot be broken into smaller parts by a chemical reaction. Some of the people who took part in the formation of the periodic table are Robert Boyle:Īround 1650, the Robert Boyle developed the definition of an element. 2 How to use a printable periodic table?.1 History of the printable periodic table:.


 0 kommentar(er)
0 kommentar(er)
